


The rate of reaction increases as you go down the group in the periodic table. The reactivity of Group 7 elements decreases down the group.
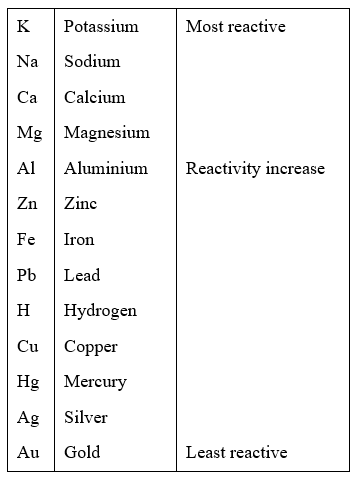
They all react quickly with oxygen in air and with water. The alkali metals are all soft metals that can be cut with a knife. It is clear from the data that we would expect reactivity to increase down the group as the energy required to remove the two outer shell electrons. This video shows a demonstration of the relative speeds of surface corrosion of alkali metals and their reaction with water. NaOHĪll alkali metal compounds are stable, this is because the alkali metals are so reactive.Īlkali metals have to be extracted from their ores by electrolysisĪlkali metal compounds are usually colourless :with water to form hydroxides and hydrogen e.g. This is because the further away an electron is from the nucleus, the weaker its attraction and the more likely it is to react with another atom. Transition metals however are not that reactive in. the attraction between the nucleus and outer electron gets weaker as you go down the group so the electron is more easily lost. Group 1 metals also react vigourously with water, this reactivity increases as you go down the group. Metals are very reactive with chemical reactivity increasing down the group. The reactivity increases down the group from Mg to Ba. Explaining trends The reactivity of Group 1 elements increases as you go down the group because: the outer electron gets further from the nucleus as you go down the group. They include lithium (Li), sodium (Na) and potassium (K). The elements in Group 1 of the Periodic Table are called the alkali metals.


 0 kommentar(er)
0 kommentar(er)
